dl
dl
When breast size or shape is an issue, many women turn to breast augmentation, or mammoplasty to increase breast size, improve symmetry, enhance appearance, and restore a confident self-image.
What We Provide:
Breast augmentation is a personal decision, and should be done for you, not to meet anyone else's expectations or ideals.
The improvement from breast augmentation will be visible immediately. Results will last a long time, but the exact duration will vary depending on health changes and natural aging. Also, augmentation is not permanent; implants may need to be replaced during your lifetime. Breast augmentation patients can return to work within a few days of surgery, though they will need to avoid physical contact with the breasts for 3 to 4 weeks. Scars should fade within several months to a year.
What to Expect
Types Of Implants
There are three basic types of implants: round gel, shaped gel, and saline-filled. Profile refers to the amount of forward projection of the implant of the chest wall. The higher profile implants create a more prominent silhouette than a lower profile implant. These options will be discussed in more detail during your consultation.
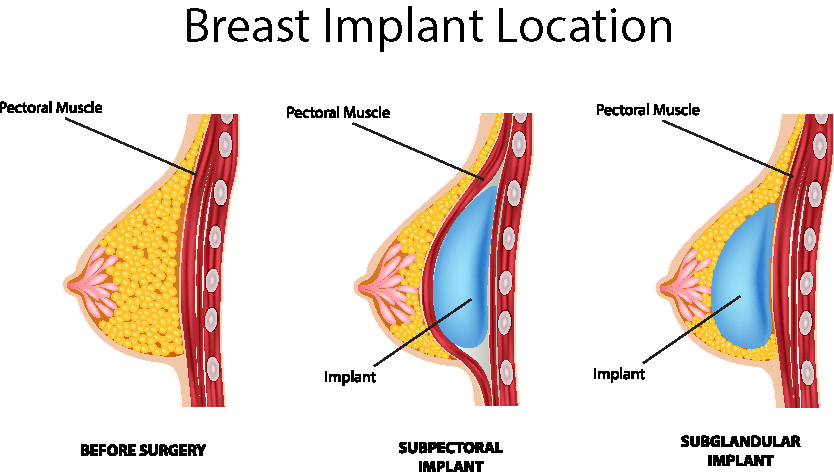
Breast Implant Placement
The breast implant can be placed either partially under the pectoralis major muscle (submuscular) or on the top of the muscle and under the breast gland (subglandular). Your surgeon will recommend the approach best-suited to your body type and goals.
Sizing your breast implant
During your office consultation, your surgeon may use different tools to help you select the right product for your new look. We use the Mentor Volume Sizing System in conjunction with your precise body measurements. Our prosthetic sizing devices are uniquely designed to be placed onto your breast to create a realistic preview of your new size.
Shaped Silicone Gel Implants
Why choose MENTOR® MemoryShape® Breast Implants?
MENTOR® MemoryShape® Breast Implants proprietary cohesive gel holds together uniformly, while providing a natural silhouette (shape) and youthful feel (firmness). Unlike round breast implants, MENTOR® MemoryShape® Breast Implants are teardrop shaped, meaning they're thinner at the top and gently slope to a fuller projection point near the implants base to mimic the silhouette of a natural breast.
Clinical data reveals MENTOR® MemoryShape® Breast Implants have the lowest reported incidence of key complications in primary augmentation at 10 years. You can also get the peace of mind you deserve knowing that MENTOR® MemoryShape® Breast Implants are covered by the most comprehensive warranty in the market.
Round Silicone Gel Implants
Our round silicone gel implants are also available as MENTOR® MemoryGel® Breast Implants and are available with either a textured or smooth surface shell. These implants are filled with a proprietary softer cohesive gel that resembles the natural feel of breast tissue. They come in a wide range of sizes and profiles to fit your body.
Saline-Filled Implants
Saline filled implants are filled with a saltwater solution similar to the fluid that makes up most of the human body. Saline implants are inserted without fluid. Once placed, the implant is filled to the predetermined size. They have a slightly firmer feel than gel implants. They are available in round and shaped, smooth and textured surfaces and are available in a full range of sizes and profiles to fit your body-each with a revolutionary self-sealing valve.
Contact Bangor Plastic and Hand Surgery today for more information or to set up a consultation with our board-certified plastic surgeon.